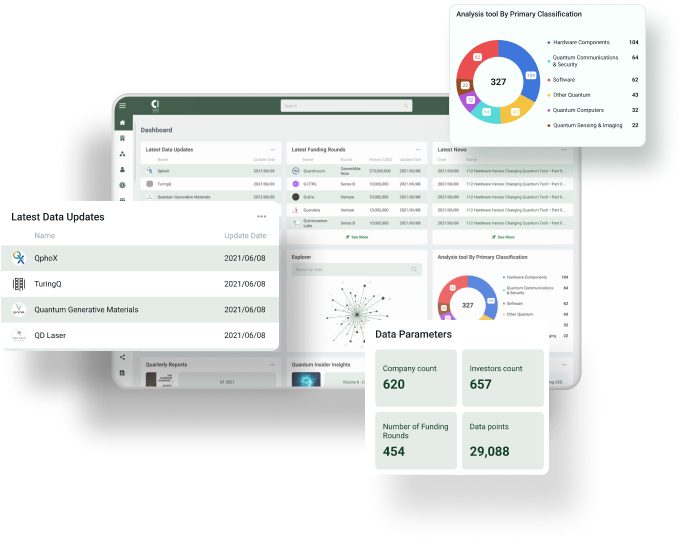
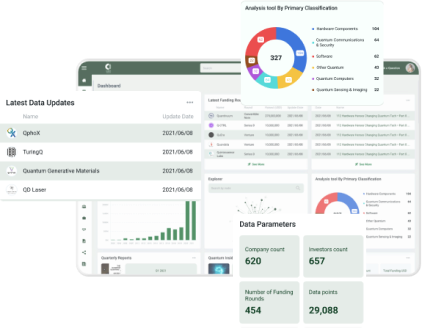
Scientists at Tokyo Institute of Technology show that copper nanoparticles within hydrophobic porous silicate crystals can significantly enhance the catalytic activity of copper-zinc oxide catalysts used in methanol synthesis via CO2 hydrogenation.

image: Tokyo Insititute of Techonology
Key Highlights:
- Scientists at Tokyo Institute of Technology encapsulated copper nanoparticles within hydrophobic porous silicate crystals.
- This encapsulation significantly enhances the catalytic activity of copper-zinc oxide (Cu-ZnO) catalysts used in methanol synthesis via CO2 hydrogenation.
- The innovative encapsulation structure effectively inhibits the thermal aggregation of copper particles, leading to increased hydrogenation activity and methanol production.
- Copper-zinc oxide (Cu-ZnO) based catalysts are favorable due to their ability to form a Cu-ZnO interface, which binds and converts CO2 into formate intermediates, promoting methanol production.
- Increasing the surface area of the Cu-ZnO interface improves production, which can be achieved by increasing the dispersion of Cu nanoparticles.
- Cu nanoparticles are thermally unstable and tend to aggregate during catalyst preparation and reaction, reducing the interface area.
- The formation of water as a byproduct of methanol synthesis accelerates Cu aggregation and inhibits formate formation.
- To address these issues, a team led by Professor Teruoki Tago developed Silicalite-1 (S-1) encapsulated Cu-ZnO catalysts.
- Encapsulating metals within porous carriers like silica or zeolite mitigates thermal aggregation of Cu particles.
- The study, supported by the EU Horizon2020 Framework and Japan Science and Technology Agency, was published in the Chemical Engineering Journal.
ICFO (the Institute of Photonic Sciences) researchers reveal the hidden power of water for green hydrogen generation.
Key Highlights:
- Hydrogen is a promising clean energy source since its utilization does not produce carbon dioxide, unlike conventional fuels.
- Currently, most hydrogen is produced from methane through a process called methane reforming, which generates substantial carbon dioxide emissions.
- To produce green hydrogen, scalable alternatives to methane reforming are necessary. Water electrolysis offers a viable method to generate green hydrogen using renewable energy and clean electricity.
- This process involves the use of cathode and anode catalysts to accelerate the otherwise inefficient reactions of water splitting and recombination into hydrogen and oxygen.
- Despite its promise, PEM water electrolysis has traditionally relied on catalysts made from scarce and expensive elements such as platinum and iridium. These materials are chosen because they can maintain activity and stability in the harsh acidic environments required for the reaction.
- Recent research by a multidisciplinary team of scientists has led to the development of an iridium-free catalyst that remains stable and active in PEM water electrolysis. This innovative approach leverages previously unexplored properties of water to confer stability and activity to the catalyst.
- The research, published in Science, was conducted by ICFO researchers alongside collaborators from several prestigious institutes, including ICIQ, ICN2, CNRS, Diamond Light Source, and INAM.
- The team employed a novel delamination process to create a cobalt-based catalyst, starting with cobalt-tungsten oxide (CoWO4). By removing tungsten oxides and replacing them with water and hydroxyl groups in a basic environment, they engineered a catalyst structure that incorporates water and water fragments.
- Using advanced photon-based spectroscopies, including infrared Raman and x-rays, the researchers studied the new catalyst material during operation. They detected trapped water within the catalyst structure, which played a crucial role in its performance.
- Looking ahead, the research team has applied for a patent and aims to scale up the technique to industrial production levels.
- The success of this research marks a significant step towards reducing reliance on iridium and other scarce elements in PEM water electrolysis.
SOURCE: EurekAlert!